
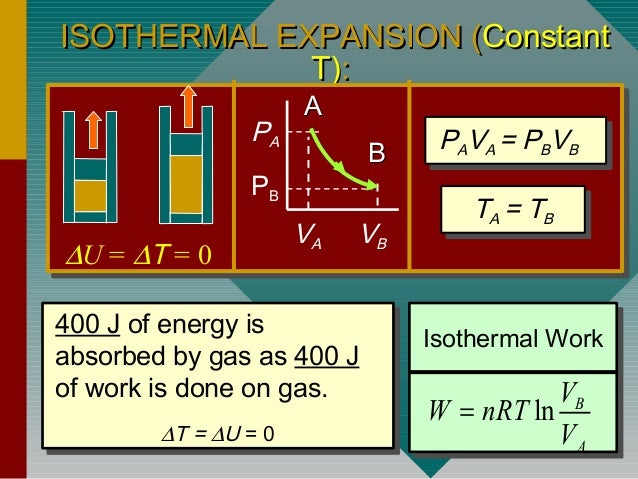
That is a variation of the state of a physical system during which the temperature of the system remains constant. Isothermal: constant temperature process (heat may flow but very slowly so that thermal equilibrium. Isothermal process is a thermodynamic transformation at constant temperature. For isothermal compression, work done is positive. W PdVwhere limits of integration goes from V1to V2Putting for PK/V, and integrating we get,W (P1V1-P2V2)/(-1)(16) In and adiabatic process if W>0 i.e. Isochoric: constant volume process (no work done). On the other hand, if the gas is compressed, the work is done on the gas is negative. done Isothermal Process Total Questions - 31 No work is done against gas Heat is relased by the gas The internal energy of gas will increase Pressure does. Need assistance Contact us on below numbers. W = ∫ 0 W d W = ∫ V 1 V 2 P d V W = \int_0^W \right) W = − 2. what is isothermal process derive an expression for the work done during yhe isothermal process h5ujd888 -Physics. isothermal, and adiabatic Both AP Physics C courses are calculus-based. Isothermal Process For a constant temperature process involving an ideal gas, pressure can be expressed in terms of the volume: The result of an isothermal. The work done is W.Īnalytically, we need to calculate between integrating dW from V 1 V_1 V 1 to V 2 V_2 V 2 . Find Work Done in Isothermal Process Calculator at CalcTown. the reversible process takes place Also, W is positive if theres work done. Figure 4.6: Work and heat exchange in the reversible isothermal compression process. Where P = pressure in N/m 2, ΔV = Change in volume.Consider that the piston with a cross-sectional area A moves a small distance dx expanding by a value dV. The work done on the system to go from state 2 to state 1 is. It is a thermodynamic process in which the pressure remains constant.įor Isobaric process the work done is given by,.A reversible adiabatic process is called isentropic.įor the adiabatic process, the work done by the system is given as.To maintain the constant temperature energy must leave the system as heat and enter the environment. Doing work on the gas increases the internal energy and will tend to increase the temperature. For the adiabatic process to occur, the system must be perfectly insulated from the surrounding. In the isothermal compression of a gas there is work done on the system to decrease the volume and increase the pressure.It is a thermodynamic process in which there is no exchange of heat from the system to its surrounding neither during expansion nor during compression, yet the temperature may change.

The process in which high-pressure fluid is converted to low pressure by using a throttle valve is Throttling. In the throttling process enthalpy remains constant, hence w ork done is zero.
#ISOTHERMAL PROCESS WORKDONE FREE#
Since for isochoric process we know that,įor free expansion work done is zero. The volume of the gas remains constant.įor constant volume or Isochoric process work done is given by,
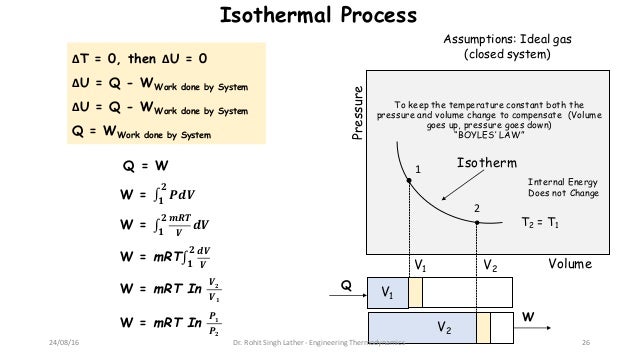
In such a process, the work done is zero.Consider pressure and volume of ideal gas changes from (P 1, V 1) to (P 2, V 2 ). It is a thermodynamic process which takes place at constant volume. Work done in Isothermal process In an isothermal process temperature remains constant.It is also known as an organized form of energy.If there is no interaction of work between system and surrounding then work done is zero.Work is said to be done by the system if the sole effect of the thing external to the system crosses the boundary.


 0 kommentar(er)
0 kommentar(er)
